

Vaccines generally combine components to achieve high bioavailability and bioactivity while eliciting a desired type and degree of immune stimulation. In the final formulation, secondary ingredients are included for transport stability and shelf-life.
The primary focus of optimal formulation development at Intravacc is the seamless alignment with a vaccine’s intended administration route. Thus, our formulations are not only remarkably stable throughout storage life, but also perfectly suited for the method of delivery. Finally, the formulation is thoroughly tested in accelerated, real-time, and in-use stability studies. These studies are conducted with a careful selection of assays that characterize the performance and longevity of the formulation and, thus, warrant the vaccine’s potency and effectiveness from production to administration.
We offer high-throughput strategies combined with a design of experiments (DoE) approach for rapid identification of vaccine formulations. When optimizing liquid formulations and lyophilization processes, we provide insights into vaccine stability via accelerated and real-time programs. In addition, the (in-use) stability of your vaccine can be assessed for a wide range of delivery routes and dosage forms.
The delivery route can be pivotal for the efficacy of a vaccine. Therefore, our experts are working on products and processes for alternative routes of administration beyond intramuscular injection, such as intranasal or mucosal sprays.
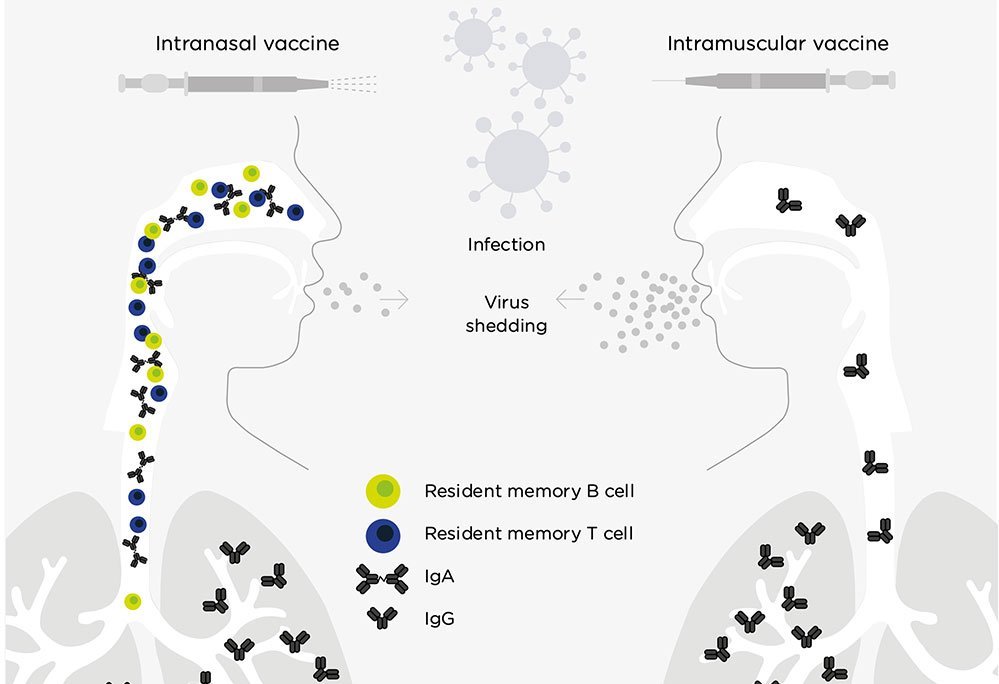
As a vaccination strategy, delivering antigen to the site of infection, in this case to the upper airways, has advantages:
The response can block subsequent infection

You can send us an email:
info@intravacc.nl
Reach out to Business Development:
BD@intravacc.nl
Or pick up the phone:
+31 30 792 03 00
You can also just fill out the contact form on the right.
We look forward to hearing from you!
