Development Services
Our CDMO Services
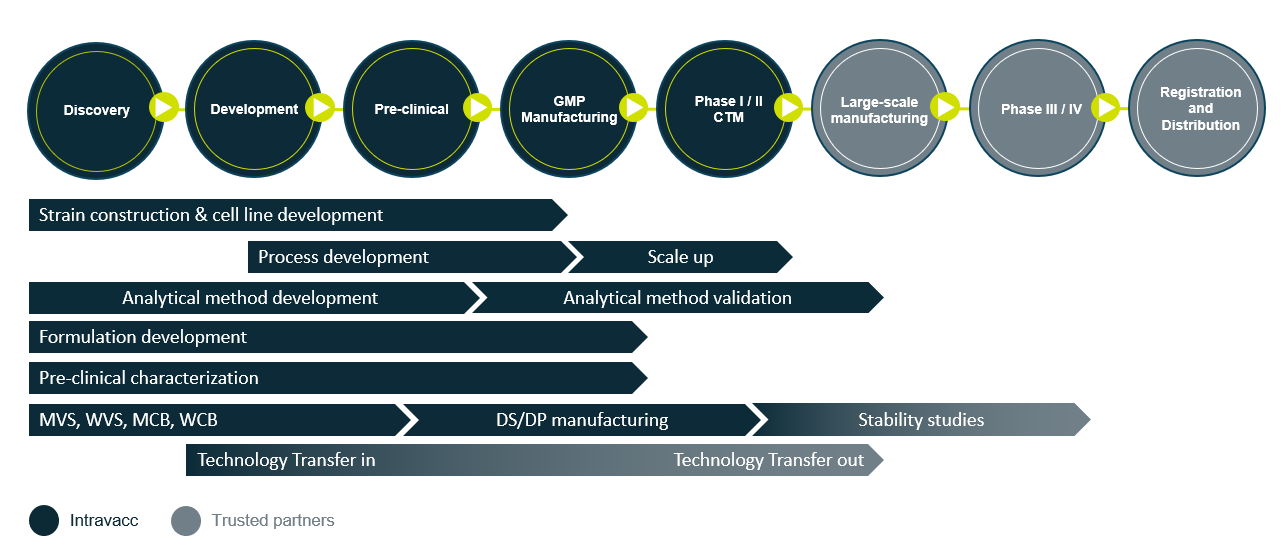
From discovery to clinical proof of concept
Expert cGMP manufacturing
From concept to clinical batches
Intravacc is specialized in guiding and managing projects from early-stage R&D through to market authorisation saving time and costs.
Whether for human or veterinary applications, we offer expertise in diverse vaccine platforms, including Viral, Bacterial, Conjugate, and Protein expression.
From Discovery to Phase II clinical trial
From early-stage R&D through to market authorisation
We are a customer-focused, global CDMO for therapeutic and infectious disease vaccines. With over 100 years of experience in the development and optimization of vaccines and vaccine technologies, Intravacc has transferred its technology to biotech and pharmaceutical companies, governmental agencies, and NGOs delivering vaccines against polio, measles, DPT, Hib, influenza, and other diseases to populations around the world.
Approximately 30% of childhood disease vaccines are based on our know-how and proprietary technology. That broad expertise; our innovative OMV, Cell, Conjugation, and E.coli expression platforms; and our state-of-the-art good manufacturing practices (GMP) facilities position us at the forefront in addressing unmet needs in the vaccine space.
Advancing vaccine development
Vaccines have revolutionized public health, helping to control, reduce, and even eliminate many serious diseases. But creating a new vaccin is a complicated process that can take 10 to 15 years.1 Intravacc has a strong track record in vaccine development and technology transfer, with highlights such as the Sabin inactivated polio (sIPV), Haemophilus influenza type B (Hib), and the Bordetella pertussis vaccines. Now we use this experience to accelerate the development of your vaccine.
Better vaccines are our goal
Working with partners worldwide, Intravacc carries out your vaccine development from lead concept to phase II clinical studies. We develop and improve the manufacturing processes of existing vaccines and produce drug substances and products at cGMP-level to enable clinical studies.
Proven strategies are our asset
Intravacc develops vaccines via established blueprints and scalable platform production processes. These processes are rationally designed using mathematical models to meet regulatory requirements and to be scalable from lab to production-scale bioreactors. Both processes and their products are tested in our state-of-the-art laboratory facilities using the latest ICH guidelines and insights.
Quality and compliance are our commitment
Our quality and regulatory affairs experts are involved at an early stage in our projects. They ensure regulatory compliance and help provide feasible solutions to challenging project needs in partnership with other company divisions.
