

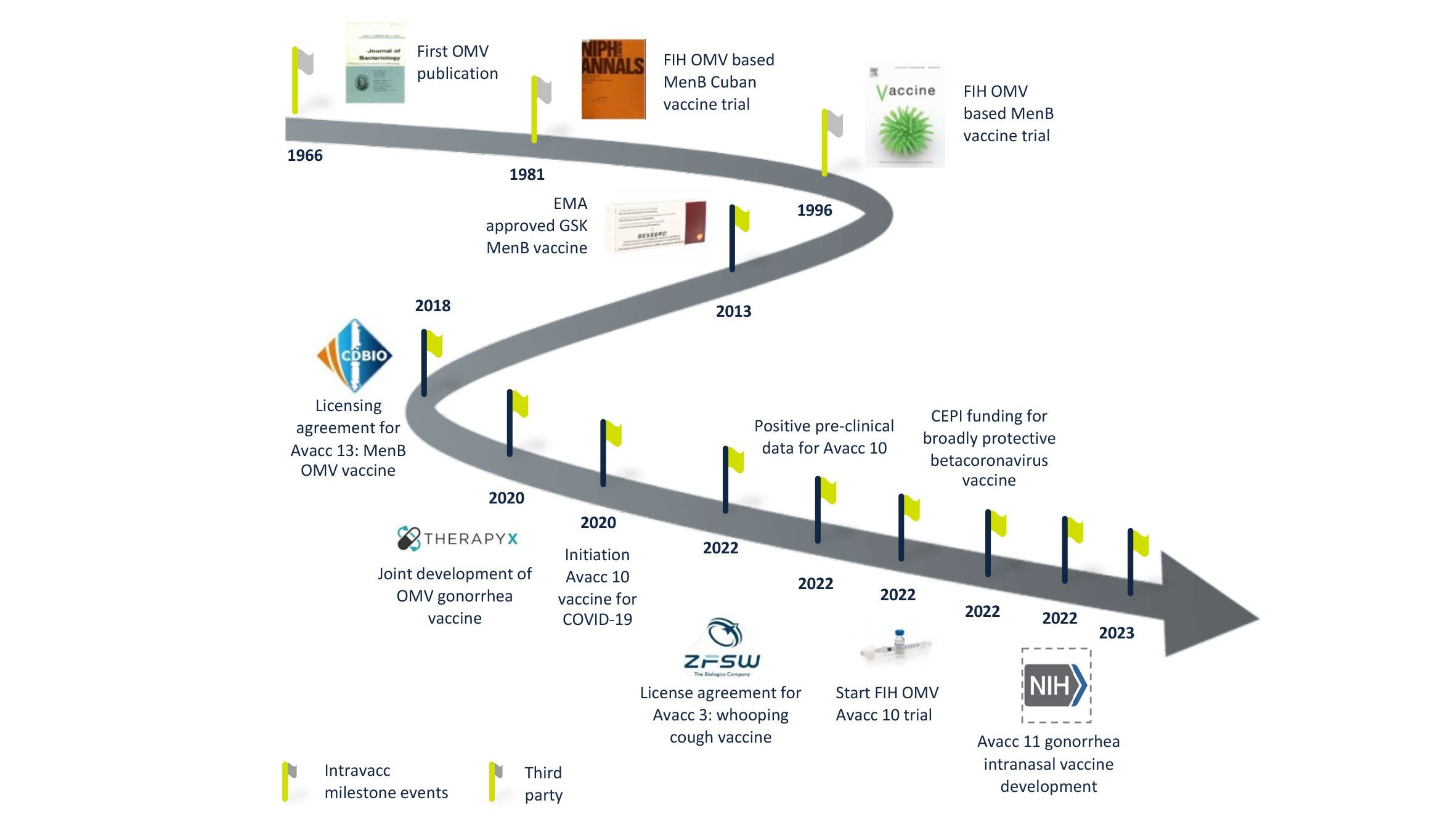
Truly exceptional among our vaccine platforms is our OMV-Vacc technology. Outer Membrane Vesicles or OMVs have been explored for vaccines since the 1980s and our experience manipulating and optimizing these membrane particles to generate effective and safe vaccines extends back over a decade. We have a number of vaccine candidates that build on this technology, which offers numerous advantages over alternative platforms, both in terms of product performance and manufacturing.
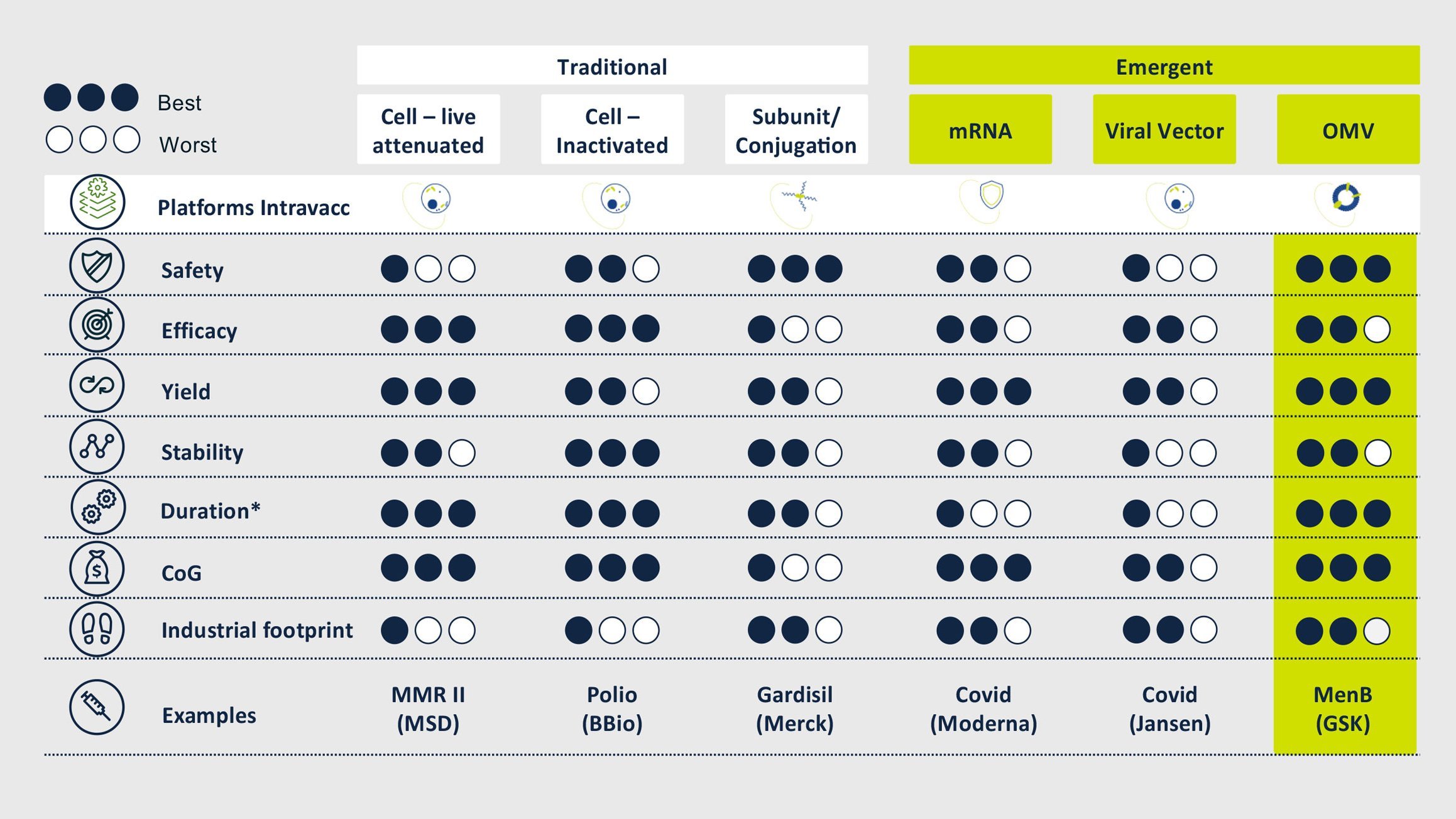
OMVs are 20-200 nm spherical particles that are naturally released by Gram-negative bacteria. They harbor many bacterial antigens and can be modified to display disease-specific antigens while increasing the safety of these vesicles by detoxification of LPS. These modified OMV have proven to be safe and intrinsically capable of stimulating both innate and adaptive immunity.
Currently, vaccine candidates are developed in one of three different modes:


The performance and safety of OMV-Vacc as a platform for vaccines is well documented. Human studies conducted by others have shown that OMVs are safe for both intramuscular and intranasal immunization. Research and development at Intravacc have led to over 80 scientific publications and nine patents. Our own investigations using OMV-Vacc span the completion of four pre-clinical candidates of which one is currently being tested in a phase I clinical study.

You can send us an email:
info@intravacc.nl
Reach out to Business Development:
BD@intravacc.nl
Or pick up the phone:
+31 30 792 03 00
You can also just fill out the contact form on the right.
We look forward to hearing from you!
