cGMP Manufacturing
Expert cGMP manufacturing
From concept to clinical batches
Comprehensive support and expertise
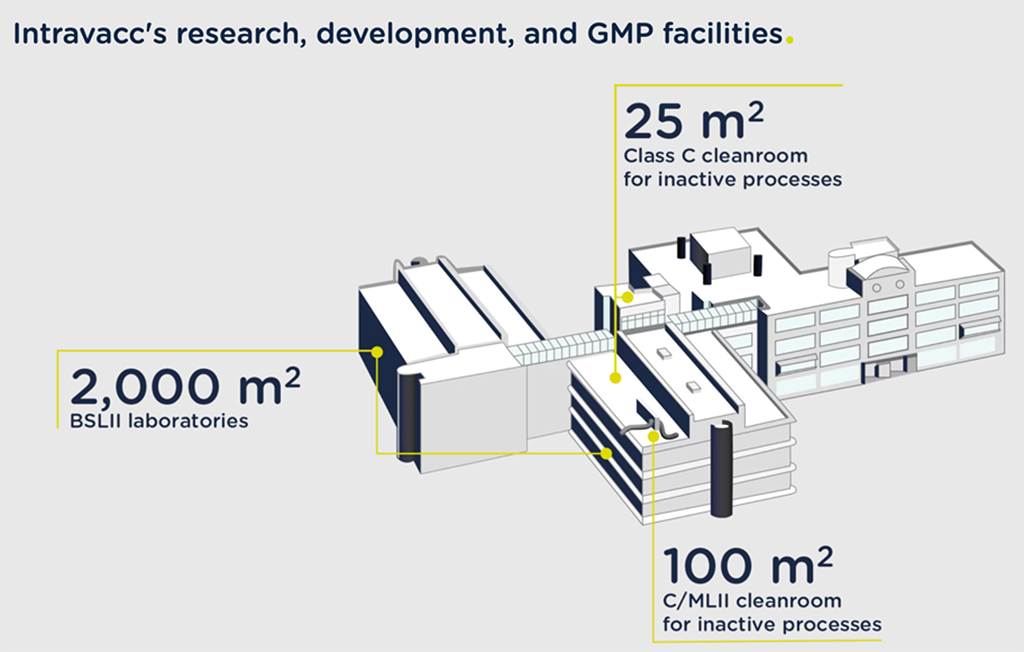
We provide comprehensive support and expertise to turn your promising research into clinical batches, whether viral, bacterial, or oncological vaccines. With a variety of production technologies in single-use and stainless-steel bioreactors with up to 200 L capacity, 230 sqm of cGMP cleanrooms (class C) and quality control, and a reliable network of service partners, we can produce vaccines for testing in phase I/II clinical studies.
Key GMP capabilities and support:
- BSL-2 clean room (class C)
- Drug substance manufacturing
- 1 to 200 L single-use bioreactors and bag systems
- DSP equipment matching defined production scales
- Technology transfer
- CMC writing for IND/IMPD through our preferred partner
Key QC capabilities and support:
- GMP-compliant QC laboratories
- Qualified QC technicians
- GMP-compliant sample management
- In-house developed and validated analytical techniques, including virus titration, protein content, immunoassays (ELISA), qPCR, HPLC, mass spectrometry, NMR, and SEC- and FFF-MALS
- Compendial assays that include pH, osmolality, and appearance
Quality control (QC) testing and stability studies
Our QC expertise includes assay validation, sampling and testing of raw materials, and management and execution of stability and release testing for your vaccine in compliance with GMP.
Accessible QC capabilities

General (microbiological) analytical techniques are outsourced to our preferred partners. These techniques include assessments of sterility, bioburden, endotoxin, mycobacteria, and mycoplasma, particulate matter subvisible, TOC and nitrates, as well as DNA fingerprinting and deep sequencing.
Assay validation services

QC includes support in the development and validation of product specific analytical techniques at the highest standards of quality. Validation of assays is performed according current IHC Q2 (R2) guidelines.
Tailor-made GMP production capabilities

With state-of-the-art manufacturing facilities and a wide range of analytical techniques, we offer focused and customized support for your vaccine development project. Furthermore, our clinical, quality affairs, and regulatory teams have the expertise needed to comply with the latest GMP standards.
